Site sections
Editor's Choice:
- White spots on the nails, reasons for what to do, white spots on the nails and folk signs
- Available methods for rapidly increasing blood leukocytes
- Nail and skin fungus will not resist the coffee grounds
- Crocus furniture exhibition. Furniture exhibitions
- Owl tattoo on arm value
- The biggest members in the world
- Fractures of the phalanges of the foot photo
- What is “bad” and “good” cholesterol
- What to do if the skin around the nails dries
- The safest natural varnishes list
Advertising
| Pathogenetic role of the inflammatory response. Inflammatory mediators: classification |
|
The appearance of inflammatory processes in response to the action of a pathological factor is an adequate response of the body. Inflammation is a complex process that develops at the local or general level, arising in response to the action of foreign agents. The main task of development inflammatory response aims to eliminate the pathological effects and restore the body. Inflammatory mediators are mediators directly involved in these processes. Briefly about the principles of inflammatory reactionsThe immune system is the guardian of human health. When necessary, it enters into battle and destroys bacteria, viruses, fungi. However, with enhanced intensification of work, the process of combating microorganisms can be seen visually or feel the appearance of the clinical picture. It is in such cases that inflammation develops as a protective response of the body. There is an acute process of inflammatory reaction and its chronic course. The first occurs as a result of the sudden action of an irritant (trauma, injury, allergic effects, infection). Chronic inflammation has a protracted nature and less pronounced clinical signs. In the case of a local response of the immune system in the zone of injury or injury, the following signs of an inflammatory reaction appear:
Stage of development of inflammationThe process of inflammation is based on the simultaneous interaction of protective factors of the skin, blood and immune cells. Immediately after contact with a foreign agent, the body responds with a local expansion of blood vessels in the zone of direct trauma. There is an increase in the permeability of their walls and increased local microcirculation. Together with the blood flow cells of humoral protection come here. In the second stage, immune cells begin to fight against microorganisms that are in the place of injury. The process called phagocytosis begins. Neutrophil cells change their shape and absorb pathological agents. Next, there are special substances aimed at the destruction of bacteria and viruses. In parallel with microorganisms, neutrophils destroy old dead cells located in the zone of inflammation. Thus, the development of the third phase of the reaction of the organism begins. The focus of inflammation as if protected from the whole body. Sometimes a pulsation can be felt in this place. Cellular inflammatory mediators begin to be produced. mast cellsthat allows you to clean the injured area from toxins, slags and other substances.
General concepts about mediatorsInflammatory mediators are active substances of biological origin, the release of which is accompanied by the main phases of alteration. They are responsible for the occurrence of inflammatory reactions. For example, increased permeability of vessel walls or a local increase in temperature in the area of trauma. The main inflammatory mediators stand out not only with the development of the pathological process. Their production occurs constantly. It is aimed at regulating body functions at the tissue and cellular levels. Depending on the direction of action, modulators have the effect:
With the appearance of damage or in the place of action of microorganisms, the mediator link controls the processes of interaction of inflammatory effectors and the change of characteristic phases of the process. Types of inflammatory mediatorsAll inflammatory modulators are divided into two large groups, depending on their origin:
Humoral inflammatory mediators are in the human body before exposure to a pathological factor, that is, the body has a supply of these substances. Their deposition occurs in cells in an inactive form. Vasoactive amines, neuropeptides, and lysosomal factors are also preexisting modulators. The remaining substances belonging to the group of cellular mediators are produced directly during the development of the inflammatory response.
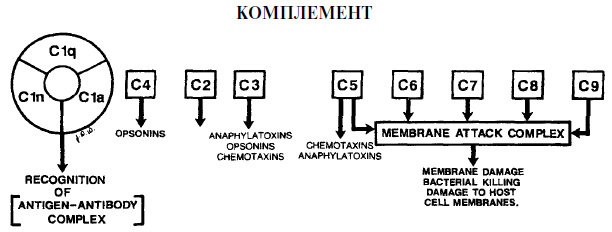
Complement derivativesThe mediators of inflammation are derivatives of the compliment. This group of biologically active substances is considered the most important among humoral modulators. Derivatives include 22 different proteins, the formation of which occurs when complement is activated (the formation of an immune complex or immunoglobulins).
This group of mediators is produced from plasma and tissue fluid. Due to the entry into the pathological zone, exudation processes occur. With the help of complement derivatives, interleukin, neurotransmitters, leukotrienes, prostaglandins and platelet activating factors are released. KininThis group of substances is a vasodilator. They are formed in tissue fluid and plasma from specific globulins. The main representatives of the group are bradykinin and kallidin, the effect of which is manifested as follows:
The action of bradykinin is aimed at opening the access of blood plasma to the site of inflammation. Kinins are mediators of pain of inflammation. They irritate the local receptors, causing discomfort, painful sensationitching. ProstaglandinsCell mediators of inflammation are prostaglandins. This group of substances belongs to arachidonic acid derivatives. Sources of prostaglandins are macrophages, platelets, granulocytes and monocytes.
Prostaglandins are inflammatory mediators exhibiting the following activity:
LeukotrienesRelated to the newly formed mediators. That is, in the body in a state of rest of the immune system, their number is not enough for an immediate response to an annoying factor. Leukotrienes provoke increased permeability of the vascular wall and open access to white blood cells in the area of pathology. Matter in the genesis of inflammatory pain. Substances can be synthesized in all blood cells, except for red blood cells, as well as in the adventitia of cells of the lung, blood vessels and mast cells. In case of development inflammatory process in response to bacteria, viruses, or allergic factors, leukotrienes cause bronchospasm, triggering the development of edema. The effect is similar to the action of histamine, but longer. The target organ for active substances is the heart. Standing out in large numbers, they act on the heart muscle, slow the coronary blood flow and increase the level of the inflammatory response. ThromboxanesThis group of active modulators is formed in the tissues of the spleen, brain cells, lung, and blood cells in platelets. They have a spastic effect on the vessels, increase the processes of thrombosis during heart ischemia, contribute to the processes of aggregation and adhesion of platelets. Biogenic aminesPrimary inflammatory mediators - histamine and serotonin. Substances are provocateurs of initial microcirculation disorders in the area of pathology. Serotonin is a neurotransmitter that is produced in mast cells, enterochromasffins and platelets. The action of serotonin varies with its level in the body. Under normal conditions, when the amount of the mediator is physiological, it increases the spasm of the vessels and increases their tone. With the development of inflammatory reactions, the number increases dramatically. Serotonin becomes a vasodilator, increasing the permeability of the vascular wall and expanding blood vessels. Moreover, its action is a hundred times more effective than the second neurotransmitter of biogenic amines.
Histamine is a mediator of inflammation that has a diverse effect on blood vessels and cells. Acting on one group of histamine-sensitive receptors, the substance dilates the arteries and inhibits the movement of leukocytes. When exposed to another, it narrows the veins, causes an increase in intra-capellar pressure and, conversely, stimulates the movement of white blood cells. Acting on neutrophil receptors, histamine limits their functionality, on monocyte receptors - stimulates the latter. Thus, the neurotransmitter can have an inflammatory anti-inflammatory effect simultaneously. The vasodilator effect of histamine is enhanced under the influence of a complex with acetylcholine, bradykinin and serotonin. Lysosomal enzymesImmune inflammatory mediators are produced by monocytes and granulocytes at the site of the pathological process during stimulation, emigration, phagocytosis, cell damage and death. Proteinases, which are the main component of lysosomal enzymes, have an antimicrobial action, lysing foreign pathological microorganisms. In addition, the active substances help to increase the permeability of the vascular walls, modulate leukocyte infiltration. Depending on the number of enzymes isolated, they can enhance or weaken the migration of leukocyte cells. The inflammatory reaction develops and persists for a long time due to the fact that lysosomal enzymes activate the complement system, release cytokines and lymokines, activate clotting and fibrinolysis.
Cationic proteinsThe inflammatory mediators include proteins contained in neutrophil granules and having high microbicides. These substances act directly on the foreign cell, breaking its structural membrane. This causes the death of the pathological agent. Next, the process of destruction and cleavage by lysosomal proteinases. Cationic proteins promote the release of the neurotransmitter histamine, increase vascular permeability, accelerate the adhesion and migration of leukocyte cells. CytokinesThese are cellular mediators of inflammation produced by the following cells:
Acting on neutrophils, cytokines increase the permeability of the vascular wall. They also stimulate leukocyte cells to kill, absorb and destroy alien colonized microorganisms, and enhance the process of phagocytosis. After killing pathological agents, cytokines stimulate the regeneration and proliferation of new cells. Substances interact with representatives from their group of mediators, prostaglandins, neuropeptides. Active metabolites of oxygenA group of free radicals that, due to the presence of unpaired electrons, are able to interact with other molecules, taking a direct part in the development of the inflammatory process. Oxygen metabolites that are part of the mediators include:
The source of these active substances are the outer layer of arachidonic acid, phagocytosis explosion during their stimulation, as well as the oxidation of small molecules.
Oxygen metabolites increase the ability of phagocytosis cells to destroy foreign agents, cause oxidation of fats, damage to amino acids, nucleic acids, carbohydrates, which increases vascular permeability. As modulators, metabolites are able to increase inflammation or have an anti-inflammatory effect. Of great importance in the development of chronic diseases. NeuropeptidesThis group includes calcitonin, neurokinin A and substance R. These are the most well-known modulators of neuropeptides. The effect of the substance is based on the following processes:
In addition to all of the above, acetylcholine, epinephrine and norepinephrine are also active mediators. Acetylcholine is involved in the formation of arterial hyperemia, dilates blood vessels in the pathology. Norepinephrine and adrenaline act as modulators of inflammation, inhibiting the growth of vascular permeability. The development of the inflammatory response is not a violation of the body. On the contrary, this is an indicator that the immune system copes with its tasks. Pathophysiology of inflammation (Lecture No. IX) Part 1. 1. The concept of inflammation. 2. Primary and secondary damage. 3. Metabolic disorders in inflammation. 4. Mediators of inflammation. 5. Stages of vascular reaction in inflammation. 6. Exudate, its types and functions. Inflammation (inflammatio) is a complex local protective-adaptive response of the connective tissue, vessels and nervous system of the whole organism, developed in the process of evolution from highly organized creatures in response to damage, is aimed at isolating and removing the damaging agent and eliminating the consequences of damage. This is a typical pathological process with changes in metabolism and blood circulation, phagocytosis and proliferation. The basis of any inflammation is: 1) damage and 2) protective reactions. The ability to resist damage, the ability to heal wounds, to restore at least some of the lost tissues is the most important property of living organisms. And these properties are determined by the fact that a healthy body responds immediately to damage by a series of general and local reactions. General reactions caused by more or less pronounced changes in the functional state of the nervous, endocrine and immune systems of the body. They are accompanied by changes in the reactivity of the whole organism. Local reactions that occur in the damage zone and in its immediate vicinity, characterize a process called inflammation. Biological meaning inflammation is to limit, delay, stop the development of damage and further, if it succeeds, clear the zone of damage from decay products and destroyed tissues, thus preparing the ground for the actual restoration processes. In the 18th century, Celsus described 4 main clinical signs of inflammation: redness (rubor), swelling (tumor), pain (dolor) and fever (calor). Galen added the fifth sign - dysfunction (functio laesa). Rubor, tumor, dolor, calor et functio laesa symptomata inflammationis sunt. Causes of inflammation : a) physical factors, b) chemical factors, c) biological factors, d) circulatory disorders, e) tumor growth, e) immune responses. Differ 4 stages: 1. alteration (alteratio) 2. exudation (exsudatio) 3. emigration (emigratio) 4. proliferation (proliferatio). Alteration- this is the main link, in fact - the trigger mechanism. Alteration can be primary or secondary. Primary Alteration develops immediately after exposure to a damaging factor and is formed at the level of the functional element of the organ. Primary alteration can manifest itself as specific changes, as well as non-specific changes that develop stereotypically regardless of the properties and characteristics of the pathogenic factor. These changes are related: 1) with damage to membrane structures, 2) with mitochondrial membrane damage, 3) with damage to lysosomes. Disruption of the cell membrane structure leads to disruption of the cellular pumps. Hence, the ability of the cell to react adequately by changing its own metabolism to changes in environmental homeostasis is lost, enzyme systems and mitochondria change. In the cell accumulate oxidized metabolites: pyruvic, lactic and succinic acids. Initially, these changes are reversible and may disappear if the etiological factor has ceased. The cell completely recovers its function. If the damage continues and lysosomes are involved in the process, then the changes are irreversible. Therefore, lysosomes are called "inflammatory launch sites" and it is from them that the formation of secondary alteration begins. Secondary alteration due to the damaging effect of lysosomal enzymes. Glycolysis, lipolysis and proteolysis are enhanced. As a result of the breakdown of proteins in tissues, the number of polypeptides and amino acids increases; Fatty acids increase with the breakdown of fats; disruption of carbohydrate metabolism leads to the accumulation of lactic acid. All this causes physical and chemical disorders in the tissues and hyperosmia develops with an increase in the concentration of K +, Na +, Ca 2+, Cl - ions; hyperkonia - an increase in the number of protein molecules due to the breakdown of large into smaller ones; Hyperionium H + - due to the dissociation of a large amount of acids with the release of hydrogen ions. And as a result of all this, metabolic acidosis develops due to an increase in acidic metabolic products. All components of the tissue are involved in the process and the alteration is irreversible, the result of which is the autolysis of cells. The formation of substances that can not only strengthen, but also weaken the alteration, affecting the various components of inflammation, i.e. regulating microcirculation, exudation, leukocyte emigration and proliferation of connective tissue cells. These biologically active substances are called mediators or inflammatory modulators. Inflammatory mediators differ ● on time their activity: early and late; ● by point of application: affecting vessels or cells and ● by origin: humoral (plasma) and cellular. Sources of inflammatory mediators can be blood proteins and extracellular fluid, all blood cells, connective tissue cells, nerve cells, non-cellular elements of connective tissue. Distinguish preformed and newly formed mediators. Preformed mediators are continuously synthesized without any damage, accumulate in special stores and are released immediately after damage (for example, histamine). Synthesis of other mediators begins after damage, as a response measure. Such mediators are called newly formed (for example, prostaglandins). Damage to the tissue is accompanied by the activation of special proteolytic blood systems, which leads to the appearance in the inflammation center of various peptides that act as inflammatory mediators. Vasoactive kinins are also formed when the fibrinolytic system is activated by activated Hageman factor, which turns inactive plasminogen circulating in the blood into an active enzyme plasmin. Plasmin cleaves fibrin (and timely digestion of fibrin is necessary for successful wound healing). At the same time peptides are formed that are able to dilate blood vessels and maintain increased vascular permeability. Plasmin activates the complement system. The complement system, which includes about 20 different proteins, is activated in addition to the Hageman factor in two more ways: the classic is the antigen-antibody complex and the alternative is the lipopolysaccharides of microbial cells. C 3a and C 5a components of the complement are involved in inflammation, which opsonize and lyse bacteria, viruses and pathologically changed own cells; contribute to the degranulation of mast cells and basophils with the release of mediators. Complement components also cause adhesion, aggregation and degranulation of blood cells, the release of lysosomal enzymes, the formation of free radicals, IL-1, stimulate chemotaxis, leukopoiesis and the synthesis of immunoglobulins. Mediators of plasma and cellular origin are interrelated and act on the principle of autocatalytic reaction with feedback and mutual amplification. Microcirculation disorder in the focus of inflammation is characterized by a change in the tone of microcirculatory vessels, enhanced current of the liquid part of the blood outside the vessel (ie, exudation) and exit uniform elements blood (i.e. emigration). For vascular response characteristic 4 stages : 1) short-term vasospasm, 2) arterial hyperemia, 3) venous hyperemia, Spasm vessels arises under the action of a damaging agent on tissue and is associated with the fact that vasoconstrictors are excited first because they are more sensitive than vasodilators. The spasm lasts up to 40 seconds and is quickly replaced by arterial hyperemia. Arterial hyperemia formed in the following three ways: ● as a result of paralysis of vasoconstrictors; ● as a result of exposure to mediators with vasodilating activity; ● as a result of the implementation of the axon reflex. The precapillary sphincters relax, the number of functioning capillaries increases, and the blood flow through the vessels of the damaged area can be ten times greater than that of intact tissue. The expansion of microcirculatory vessels, an increase in the number of functioning capillaries and an increased blood supply to the body determines the first macroscopic sign of inflammation - redness. If inflammation develops in the skin, the temperature of which is below the temperature of the blood flowing, the temperature of the inflamed area increases - there is a fever. Since at the first time after injury, the linear and volumetric blood flow velocity in the area of inflammation is large enough, the blood flowing from the source of inflammation contains more oxygen and fewer recovered hemoglobin and therefore has a bright red color. Arterial hyperemia during inflammation does not last long (from 15 minutes to an hour) and always turns into venous hyperemia, in which the increased blood supply to the body is combined with slowing down and even complete cessation of capillary blood flow. Venous hyperemia begins with the maximum expansion of precapillary sphincters, which become insensitive to vasoconstrictor stimuli and venous outflow is hampered. After that, the blood flow in the capillaries and the arterioles is slowed down. The main reason for the development of venous hyperemia is exudation - the exit of the liquid part of the blood from the microvasculature into the surrounding tissue. Exudation is accompanied by an increase in blood viscosity, peripheral resistance to blood flow increases, the speed of blood flow decreases. In addition, the exudate compresses the venous vessels, which complicates the venous outflow and also increases venous hyperemia. The development of venous hyperemia is promoted by swelling in the acidic environment of the blood corpuscles, thickening of the blood, impaired desmosomes, marginal standing of leukocytes, the formation of microthrombi. The blood flow gradually slows down and acquires new qualitative features due to an increase in hydrostatic pressure in the vessels: the blood begins to move jerkly when the blood moves forward at the time of systole of the heart, and at the time of diastole the blood stops. With a further increase in hydrostatic pressure, the blood in the systole moves forward, and at the time of diastole it comes back - that is, the pendulum-like movement arises. Push and pendulum movement of blood determines the occurrence of pulsating pain. Gradually exudation causes the development of stasis - a common phenomenon in inflammation. Usually, stasis occurs in individual vessels of the venous part of the microcirculatory bed due to a sharp increase in its permeability. At the same time, the liquid part of the blood quickly passes into the extravascular space and the vessel remains filled with a mass of blood cells that are closely related to each other. The high viscosity of such a mass makes it impossible to move it through the vessels and stasis occurs. Red blood cells form "coin columns", the boundaries between them are gradually erased and a solid mass is formed in the lumen of the vessel - sludge (from the English. Sludge - Tina, dirt). Exudation Mechanisms: exudation due to inflammation is primarily due to the increased permeability of the microvasculature for protein as a result of a significant change in the vascular endothelium. Changes in the properties of the endothelial cells of microcirculatory vessels are the main, but not the only cause of exudation during inflammation. The formation of various exudates contributes to the growth of hydrostatic pressure inside the microcirculatory vessels, associated with the expansion of the bringing arterioles, an increase in the osmotic pressure of interstitial fluid, due to the accumulation of osmotically active tissue degradation products in the extravascular space. More significantly, the process of exudation is expressed in the venules and capillaries. Exudation forms the fourth sign of inflammation - swelling (tumor). Exudate composition (exsudatum) is the liquid part of the blood, the formed elements of the blood and the destroyed tissues. The composition of the exudate emit 5 types of inflammation: ● serous; ● catarrhal (mucous); ● fibrinous; ● hemorrhagic; ● purulent; ● ichorous. Exudate functions - as a result of exudation, the concentration of bacterial and other toxins is diluted and destroyed by proteolytic enzymes coming from blood plasma. During exudation, serum antibodies enter the focus of inflammation, which neutralize bacterial toxins and opsonize bacteria. Inflammatory hyperemia provides a transition to the focus of inflammation of blood leukocytes, promotes phagocytosis. Fibrinogen exudate turns into fibrin, the threads of which create a structure that facilitates the transition of leukocytes into the wound. Fibrin plays an important role in the healing process of wounds. However, exudation also has negative consequences - tissue swelling can lead to choking or an increase in intracranial pressure that threatens life. Disorders of microcirculation can lead to ischemic tissue damage. Excessive deposition of fibrin may impede the subsequent repair of damaged tissue and contribute to excessive proliferation of connective tissue. Therefore, the doctor should exercise effective control over the development of exudation. Pathophysiology of inflammation (Lecture No. X) Part 2. 1. Emigration of leukocytes in the focus of inflammation. 2. Functions of leukocytes in the focus of inflammation. 3. Acute and chronic inflammation. 4. The biological essence of inflammation. 5. Diagnosis of inflammation. When the arterial hyperemia passes into the venous leukocytes, they gradually move from the axial layer to the peripheral - parietal layer and begin to adhere to the endothelium surface. "Leukocyte marginal standing" appears and from this moment begins the mass migration of leukocytes to the inflammation center. The leukocyte must overcome two barriers: the endothelium and the basement membrane. The endothelium layer of leukocytes pass, squeezing between the endothelial cells, and the basement membrane is temporarily dissolved by its proteases. The whole process of transition of the leukocyte through the vessel wall takes from 2 to 12 minutes and does not cause damage to the vessel wall. The main place of emigration of leukocytes are postcapillary venules. In acute inflammation, neutrophils first of all emigrate and, much later, monocytes. Eosinophils, basophils and lymphocytes are also capable of emigration. Leukocyte emigration is associated with the emergence of special mediators hemattractants in the inflammatory focus. The strongest hemattaractants are lipopolysaccharides, which are part of bacterial endotoxins. The most powerful endogenous hemattractant includes fragments of the complement activated during inflammation, especially C5a, leukotriene B4, platelet activating factor and calicrein. The emigration of leukocytes into the inflammation center begins with their adhesion to the vascular endothelium of the microvasculature. Adhesiveness increases as a result of the increased formation of endothelial cells of special RNA molecules and their corresponding protein. The passage of leukocytes through the vascular wall is the result of the ability to move inherent in these cells - i.e. locomotionwhich is also activated by hemattractants. Inside the cytoplasm of leukocytes, the concentration of calcium ions increases. This activates the microtubule system, which forms the inner skeleton of the cell, activates actomyosin complexes, the secretion of neutrophils of their granular contents, including neutrophilic proteases capable of dissolving the basement membrane of blood vessels, is enhanced. The interaction of hemattractants with surface receptors of leukocytes is accompanied by the activation of various enzymes in them, including calcium-dependent phospholipase A2, calcium-dependent protein kinases: protein kinase A and protein kinase C. Under the influence of hemattractants in the leukocyte at the front pole, the cortical gel turns into a sol, i.e. becomes more liquid. The sol of its central part is poured into this diluted part of the leukocyte. The leukocyte is shortened in the back and lengthened in front. The liquefied part of the cortical gel of the anterior pole is thrown back with force and thus the leukocyte moves forward. Neutrophilic leukocytes have the greatest functional activity. Polymorphonuclear leukocytes are the first to come to the focus of inflammation because they are more sensitive, they are much more in the blood. They are called cells. " emergency response"and disposable. Monocytes are in the blood for up to 3 days, go to the tissue and are in them for about 10 days. Some of them are differentiated into sedentary tissue macrophages, some are inactive and can be re-activated. Therefore, monocytes are called reusable cells. Such a sequence of the release of blood cells outside the vessel has been identified by Mechnikov and is called the “law of emigration” or “stage of cellular reaction during inflammation”: 1) polynuclear (neutrophils and eosinophils) up to 2 days, 2) mononuclear (monocytes and lymphocytes) up to 5-6 days, 3) fibroblastic, characterized by the accumulation of histiocytes and fibroblasts in the focus of inflammation. The most important function of leukocytes in the focus of inflammation is phagocytosis - i.e. capture, killing and digesting bacteria, as well as the digestion of the decay products of tissues and cells of the body. During phagocytosis, there are 4 stages : 1) the stage of phagocyte approximation to the object; 2) the stage of adherence of the phagocyte to the object; 3) the stage of phagocyte absorption of the object; 4) the stage of intracellular transformations of the absorbed object. The first stage is explained by the ability of phagocytes to chemotaxis. Opsonins, antibodies and complement fragments, plasma proteins and lysozyme, play a large role in the mechanisms of sticking and subsequent absorption by the phagocyte of an object. It has been established that certain parts of the opsonin molecules bind to the surface of the attacked cell, and other parts of the same molecule - with the phagocyte membrane. The mechanism of absorption does not differ from sticking - capture is carried out by gradually enveloping the microbial cell with a phagocyte, i.e. essentially by progressively sticking the surface of the phagocyte to the surface of the microbe until the entire object is glued to the phagocyte membrane. As a consequence, the absorbed object is inside the phagocyte, enclosed in a bag formed by part of the membrane of the phagocytic cell. This bag is called a phagosome. Phagosome formation begins the stage of intracellular transformations of the absorbed object inside the phagosome, i.e. outside the internal environment of the phagocyte. The main part of the intracellular transformations of an object absorbed during phagocytosis is associated with degranulation — that is, the transfer of the contents of cytoplasmic granules of phagocytes into the phagosome. In these granules, all obligate phagocytes contain a large number of biologically active substances, mainly enzymes, which kill and then digest microbes and other absorbed objects. In neutrophils, there are 2-3 types of granules that contain lysozyme - dissolving the microbial wall, lactoferrin - a protein that binds iron and thus has a bacteriostatic effect, myeloperoxidase, neutral proteases, acid hydrolases, a protein that binds vitamin B 12 and others. As soon as a phagosome is formed, granules approach it closely. The granule membranes merge with the phagosome membrane and the contents of the granules enter the phagosome. As already mentioned, neutrophils are the first leukocytes infiltrating the area of inflammation. They provide effective protection against bacterial and fungal infections. If the wound is not infected, then the content of neutrophils in it quickly decreases and after 2 days macrophages predominate in the focus of inflammation. Like neutrophils, inflammatory macrophages are motile cells that protect the body by phagocytosis from various infectious agents. They are also able to secrete lysosomal enzymes and oxygen radicals, but differ from neutrophils by a number of qualities that make these cells especially important in the later stages of acute inflammation and in the mechanisms of wound healing: 1. Macrophages live much longer (months, and neutrophils - a week). 2. Macrophages are able to recognize and then absorb and destroy damaged and non-viable cells of their own organism, including neutrophils. Related to this is their extraordinary role in the "cleaning" of inflammatory exudate. Macrophages are the main cells involved in the dissolution and removal of damaged connective tissue from the focus of inflammation, which is necessary for subsequent tissue reconstruction. They synthesize and secrete neutral proteases: elastase, collagenase, plasminogen activator, destroying extracellular collagen and elastin fibers of connective tissue. Macrophages play a key role in wound healing. In animals in the experiment, devoid of mononuclear cells, wounds do not heal. This is explained by the fact that macrophages synthesize growth factors for fibroblasts and other mesenchymal cells, produce factors that increase collagen synthesis by fibroblasts, are sources of factors that control various stages of angiogenesis — revascularization of damaged tissue, produce polypeptide hormones that mediate the acute phase response — interleukin -1 and IL-6 and tumor necrosis factor. Inflammation is divided into acute and chronic. Sharpinflammation (inflammatio acuta) develops due to sudden damage - burn, frostbite, mechanical injury, some infections. Its duration usually does not exceed several days. Acute inflammation is characterized by pronounced exudative reactions, during which water, proteins, blood cells (mostly leukocytes) leave the bloodstream and enter the damaged area. Chronic inflammation (inflammatio chronica) develops when the damaging agent acts for a long time. Chronic inflammation lasts weeks, months and years. It is characterized not so much by exudation as by the proliferation of fibroblasts and vascular endothelium, as well as by the accumulation of special cells in the focus of inflammation - macrophages, lymphocytes, plasma cells and fibroblasts. Most of the most serious human diseases are characterized by a chronic inflammatory process - leprosy, rheumatoid arthritis, tuberculosis, chronic pyelonephritis, syphilis, liver cirrhosis, and so on. Chronic inflammation is usually accompanied by irreversible damage to the normal parenchyma, the defects of which are filled with fibrous connective tissue that deforms the affected organs. In the optimal case, the cessation of the action of the damaging agent is accompanied by the attenuation of the inflammatory response and the complete elimination of all the consequences of the inflammatory reactions themselves - i.e. "full resolution of inflammation". This means the cessation of the formation of mediators and their disappearance from the damage zone, the cessation of the emigration of leukocytes, the restoration of vascular permeability, the removal of fluid, proteins, breakdown products of bacteria and cells (including neutrophils and macrophages). The disappearance of mediators is due in part to their spontaneous diffusion from the source of inflammation and partially inactivation by various enzymes, with the inactivation system developing during the course of inflammation itself. If the increase in vascular permeability was not associated with gross damage to the endothelial cells, then the permeability is quickly normalized after the disappearance of the mediators. Most of the inflammation accumulated in the nidus is removed with lymph flow. Fibrin deposits are dissolved by blood fibrinolytic enzymes, inflammatory cell enzymes and are also removed by lymphatic vessels. It is possible that macrophages also leave the lymphatic vessels. Part of macrophages loaded with non-toxic intact substances can remain for a long time in the place of the former inflammation. Full resolution of inflammation creates the conditions for full restoration of the structure and function of damaged tissues. However, this happens only with relatively small wounds of organs and tissues that also have a high capacity for regeneration - the skin, mucous membranes, and parenchyma of the internal organs. Incomplete resolution of inflammation leads to the fact that recovery occurs through scarring. General reaction of the body inflammation depends on the location, cause, degree of damage to the organ, the occurrence of insufficiency of the organ, reactivity and resistance of the body, immunity, state of the endocrine glands, nutrition, constitution, sex, age, previous diseases. The biological essence of inflammation. I.I. Mechnikov, 25 years old (since 1882), has investigated phagocytosis. His method of comparative pathology is the study of the process in an evolutionary aspect. He proved that inflammation occurs in all members of the animal world. In unicellular protection and nutrition are the same. In the lower multicellular (sponge), all cells can phagocytose. During the formation of germ layers, phagocytosis is attached to the mesoderm. When an open-type vascular system (crayfish) is formed, phagocytes are more easily delivered to the inflammatory focus and, in the higher ones, the reaction of the vessels, nervous system and connective tissue joins the phagocytic reaction. This is a reaction of the whole organism, developed in the process of evolution, has a protective and adaptive value - phagocytosis is the basis of protection, all the rest is just accessories of the inflammatory reaction. Diagnosis of inflammation - on the visible areas of the tissue, it is manifested by the above symptoms: redness, fever, swelling, pain and impaired function. Evaluation methods functional evaluation of phagocytes: a) determination of the functional activity of leukocytes: 1.% of phagocytosis is an extensive indicator of% of phagocytic cells per 100 potential phagocytes, 2. The phagocytic number is the number of phagocytosis objects captured by these 100 phagocytes, 3. phagocytic index - or the intensity of absorption - is the number of captured objects of phagocytosis, which accounts for each phagocytic leukocyte, 4. total absorption intensity is the number of phagocytosis objects captured by phagocytes contained in 1 mm 3, 5. completeness of phagocytosis, 6. conglomerate index - the rate of disappearance of coarse dye from the blood when administered intravenously after repeated studies of venous blood in 15-20 minutes, 7. to assess the degree of vaccination determine the antibody titer, 8. The cellular composition of the exudate is investigated. 9. Determination of the total number of leukocytes and leukocyte formula. The dependence of the inflammatory reaction on the general condition - reactivity and resistance, which provide the appearance, development, course and outcome of inflammation. Inflammation can be: ● normergic - with good reactivity in healthy individuals, ● Hyperergic (very rapid) - in case of allergies or in choleric individuals, Inflammation- it is a phylogenetic protective pathological process arising in response to tissue damage, which includes characteristic alterative, microcirculatory and proliferative changes, ultimately aimed at isolating and eliminating the damaging agent, dead tissue, and more or less complete organ recovery. Celsus described 4 prizes of inflammation: redness (rubor), fever (calor), swelling (tu-mor), pain (dolor). Galen added to them the fifth sign - the violation of the function (functio laesa). In addition to these, there may be the following common symptoms inflammations: leukocytosis, fever, changes in the protein, hormonal and enzymatic composition of the blood, increased ESR, etc. The dynamics of the inflammatory process, regardless of the reasons causing it, is always fairly standard. There are 3 components of inflammation: alteration, microcirculation disorder, and hemorheology with exudation and leukocyte emigration, proliferation. Alteration(damage) is a violation of the structural and functional organization of cells and the intercellular substance of tissues and organs, which is accompanied by a violation of their vital activity. It is customary to distinguish primary and secondary alterations. Primary alteration occurs in response to the direct effect of the factor causing inflammation. The reactions of the primary alteration as if prolong the action of the damaging factor. The factor itself may no longer be in contact with the body. Secondary alteration occurs under the influence of both the factor causing inflammation and the factors of primary alteration. The effect of the damaging factor is manifested primarily on the cell membranes, including lysosomal ones. Lysosome enzymes are reactive. They go outside and damage all the elements of the cell. Thus, secondary alteration is primarily self-harm. At the same time, the secondary alteration is quite a reasonable and necessary component of inflammation - as a protective and adaptive process. Additional counter damage is aimed at the early localization of the etiological factor and the tissue of the body damaged under its influence. At the price of damage, many other important protective phenomena are achieved: activation of the metabolism, involvement of inflammatory and cellular mediators, increased phagocytosis, etc. Metabolic change at the onset of inflammation occurs predominantly due to carbohydrate. Initially, due to the activation of tissue enzymes, both oxidative phosphorylation of carbohydrates and glycolysis are enhanced. Subsequently, glycolysis begins to predominate over respiration. This is because: 1. Increased consumption of oxygen by the inflamed tissue. 2. Blood circulation is disturbed. The blood decreases the oxygen content. 3. The accumulation in the lesion of leukocytes, lysosomal enzymes, which break down glucose mainly anaerobically, increases. 4. Damage and reduction in the number of mitochondria occurs. Under-oxidized carbohydrate metabolism products accumulate in fabrics: lactic and tricarboxylic acids. Violation fat metabolism lies in the fact that under the action of enzymes primarily lysosomal in the outbreak acute inflammation Fats break down to form fatty acids. In the outbreak of inflammation sharply disturbed protein exchange and nucleic acids. Under the action of lysosomal and other enzymes, the breakdown of proteins and nucleic acids to amino acids, polypeptides, nucleotides, nucleosides (adenosine) occurs. As a result of metabolic disorders of carbohydrates, fats and proteins, acidic metabolic products accumulate in the inflamed tissue and develops metabolic acidosis. At the beginning, it is compensated by alkaline reserves of blood and tissue fluid. In the future, with the local depletion of alkaline reserves and with the difficulty of the inflow of fresh blood, the acidosis becomes uncompensated. With acute purulent inflammation pH can reach 5.4, and in chronic - 6.6. Aci-doses create favorable conditions for the action of certain lysosomal enzymes, in particular glycosidases, which break down the carbohydrate components of connective tissue. The concentration of hydrogen ions increases the more, the more intense the inflammation flows. In the direction from the center to the periphery, the concentration of hydrogen ions gradually decreases. In an acidic environment, the dissociation of salts increases. As a result, the content of K, Na, Ca ions increases in the inflammation focus. This is also due to the destruction of cells and the release of these salts. Due to the reduced formation of macroergs, the potassium-sodium balance in the cell is disturbed. Potassium begins to leave the cells, sodium, on the contrary, enters the cell. Hyperionium and di-zionia appear. At the same time, the molecular concentration increases, as in the process of tissue breakdown and impaired metabolism, large molecules break down into many small ones. Due to an increase in ionic and molecular concentration, hyperosmia develops. Hyperkonia leads to hyperosmia - an increase in protein concentration in the inflammation focus. Hyperconia occurs because: 1) protein is released from the blood to the inflammatory focus, due to the fact that acidosis and lysosomal enzymes increase the permeability of the vascular wall to the protein; 2) under the conditions of acidosis, the fission of coarse proteins to fine. Inflammatory mediators Mediators/ intermediaries / inflammations - it is a complex of physiologically active substances mediating the action of factors causing inflammation and determining the development and outcomes of inflammation. During inflammation, they are excreted in large quantities and become mediators. Because they are able to strengthen or weaken the manifestation of the inflammatory process they are called modulators. The mediator link is important in the pathogenesis of inflammation. The main groups of inflammatory mediators are: 1. Biogenic amines - histamine, serotonin. Histamine, one of the most important mediators, is secreted by basophils and mast cells and realizes its action through membrane receptors. Histamine release is one of the first tissue re-actions to damage. Histamine causes vasodilation, increases vascular permeability due to rounding of endothelial cells and weakening of intercellular contacts, increases the production of pro-taglandin E 2, reduces the release of lysosomal enzymes, neutrophils. Have a person appear pruritus, burning and pain. After release, histamine is very rapidly destroyed by the enzyme histaminase. Therefore, its action quickly stops and other mediators, in particular serotonin, are switched on. It is contained in the neuro-brain, basophils, platelets. In the focus of inflammation, serotonin in moderate doses causes dilation of arterioles, reduction of myocytes in the walls of venules, and venous congestion. In addition, it increases the permeability of the vascular wall, increases blood clots, causes a feeling of pain. Biogenic amines interact between themselves and other inflammatory mediators. For example 2nd group of mediators: plasma systems / kinins, complement, components of the component system, blood coagulation factors /. The most important kinins are bradykinin and kallidin. The starting point of the activation of the kinin system is the activation of 12 coagulation factor - Hageman factor in case of tissue damage. This factor turns prekallikreiny in kallikreiny. The latter act on plasma protein kininogen and plasmaquinines are formed from it. They cause dilation of arterioles and increase the permeability of venules, reduce the smooth muscle of the veins, and increase the blood pressure. Kinins inhibit neutrophil emigration, stimulate the migration of lymphocytes, the secretion of lymphokines, and cause a feeling of pain. Complement is a complex plasma system comprising at least 18 proteins. It provides lysis of foreign and native altered cells. Complement fragments can increase vascular permeability, release lysosomal hydrolases, participate in the formation of leukotrienes. The system of hemostasis and fibrinolysis promotes thrombosis and the formation of fibrinopeptides. They increase the permeability of blood vessels, stimulate the formation of kinins. The 3rd group of mediators are products of arachidonic acid — prostaglandins and leukotrienes. PGs are produced by almost all types of nuclear cells, but predominantly by leukocytes. PGs enhance or weaken the action of other mediators, inhibit or increase platelet aggregation, dilate or dilate blood vessels, and increase body temperature. Leukotrienes are formed in the membranes of platelets, basophils, endothelial cells. They cause leukocyte aggregation, microvascular spasm, increased permeability, bronchospasm. The 4th group of mediators - oxygen radicals and lipid hydroperoxides. In the mitochondria of cells, oxygen radicals such as hydrogen peroxide, hydroxyl radical, etc. are formed. When mitochondria are damaged, acidic radicals are released, interacting with membrane lipids, forming lipid hydroperoxides. The whole complex of processes for the generation of oxygen radicals and lipid hydroperoxides is called the "oxidative system". In the focus of inflammation, free radical processes are activated and damage the membranes of microbial and own cells. A so-called "oxidative explosion" arises. It is the basis of the bactericidal activity of phagocytes. In addition, radicals increase the permeability of microvessels, can stimulate proliferation. The 5th group of mediators is a mediator of polymorphonuclear leukocytes / PMN / monocytes and lymphocytes. PMNs emit a group of highly active mediators that cause various reactions in the inflammatory focus, forming its manifestations. One of the representatives is platelet activating factor / PAF /. It increases the permeability of vessels, causes platelet aggregation, leukocyte emigration. In addition, leukocytes secrete mediators such as prostaglan E 2, leukotrienes, thromboxane A 2 (increases blood clotting, narrows coronary vessels), prostacyclin (expands blood vessels and reduces blood clotting). Prostacyclins and leukotriens are important in the origin of inflammatory pain. Mono-cytes and lymphocytes secrete monokines and lymphokines. For example, lymphocytes secrete a factor that inhibits macrophages, macrophage-stimulating factor. Lymphokines coordinate the interaction of neutrophages, microphages and lymphocytes, regulating the inflammatory response in general. Antimediators of inflammation At all stages of inflammation, substances that prevent excessive accumulation of mediators or stop the influence of mediators are released and act. These are primarily enzymes: histaminase, carboxypeptidase kinin inhibitors, esterase inhibitors of the complement fraction. Eosinophils play an important role in the formation and delivery of anti-medications to the inflammatory focus. Of humoral anti-mediators, an important role is played by alpha-1-antitrypsin, which is formed in hepatocytes. It is a protease inhibitor. As follows from the definition The 2nd component of inflammation is a violation of microcirculation and hemorheology in the inflammation focus. The following stages of circulatory disorders are distinguished: 1. Formation of arterial hyperemia. 2. Stage of venous hyperemia, which passes through the mixed. 3. Next can come blood stasis. Quickly formed histamine, kinins, prostaglandins and other inflammatory mediators dilate arteries, arterioles and ensure the formation of arterial hyperemia. An important role in the development of the arterial hyperemia and its maintenance belongs to the change in the sensitivity of the alpha-adrenoreceptors of the vessels in aci-dose conditions. As a result, there is a decrease in the reaction of the vessels to adrenalin and sympathetic influences, which contributes to the expansion of arteriole and precapillary sphincters. In the focus of inflammation due to acidosis, dysionia (increased concentration of K + ions in the tissue fluid), the vasoconstrictive effect of precapillary sphincters also decreases. All these factors lead to the formation of arterial hyperemia. Arterial hyperemia is characterized by an increase in the volume and linear velocity of blood flow, the number of functioning capillaries. An increase in the flow of blood rich in oxygen contributes to the enhancement of redox processes and heat generation. Therefore, in the stage of arterial hyperemia, an increase in temperature in ocular inflammation is subjectively and objectively recorded. When inflammation increases the permeability of blood vessels, which contributes to the release of protein and water into the focus of inflammation. First of all, there are albumin, in connection with which the amount of globulins and fibriogen increases in blood. This entails an increase in viscosity and blood concentration, the result is a slowing down of blood flow and the formation of aggregates of red blood cells. As a result of the accumulation of fluid, and later the formed elements in the tissue, lymphatic and blood vessels are compressed, which makes it difficult for the blood and lymph to flow out. Aggregation of uniform elements, their pasting and sludge formation develops in the vessels. For sweets, erythrocyte aggregation in the form of coin columns is characteristic. With a sweeter, the erythrocyte membrane does not break up, so sludge can break up. In parallel with this, the blood coagulation system is activated with the formation of blood clots and thromboembolism. All these changes contribute to the increase of the dynamic viscosity of the blood and the deterioration of its rheological properties. Also, the cause of microthrombus formation and hemorrhage is direct damage to the vascular wall, a factor causing inflammation, activation of Hageman factor, action of mediators / lysosomal enzymes, bradykinin, kallidin /. Red blood cells leave the vessels through the interendothelial spaces. Thus, the arterial hyperemia very quickly joins the venous, the manifestations of which progressively increase. In the stage of venous hyperemia, the outflow of blood from the source of inflammation is disturbed, the linear and volumetric velocity of the blood flow decreases, the hydrostatic pressure increases, and the jog-shaped and pendulum-like blood flow develops. With the development of inflammation and venous hyperemia, a further, progressive slowing down of blood flow occurs. It is caused by: a) an excessive increase in the cross-sectional area of the vascular dorsum due to the maximum dilation of the capillaries and opening of the veins, b) a mechanical obstacle to the outflow of blood and lymph from the focus of inflammation, primarily due to compression of the venous and lymphatic vessels, ) an increase in the resistance to blood flow due to the roughness of the inner wall of small vessels from leukocytes adhering to it, as well as swelling of endothelial cells; d) further thickening of the blood and increasing its viscosity due to increased about the release of fluid from the vessels into the tissue. In the end, there is a stop of blood movement - stasis. Stasis is initially recorded in separate capillaries and venules, later it covers more and more vessels. After all, stasis develops in aterioles. Depending on the severity of inflammation, stasis can be short-lived, persist for hours or be irreversible. The consequence of stasis can be irreversible changes in blood cells and tissues. Exudation Exudation - this is the exit of the liquid part of the blood into the focus of inflammation. It is carried out in 3 ways: 1. Through interendothelial slits, the size of which increases due to the reduction of microfibrell endothelial cells. 2. Through the body of endothelial cells through specialized channels. 3. Micropinocytosis pathway in the form of actively conducting the smallest drops through the cell body. Two phases of increasing the permeability of the vascular wall in the inflammatory focus have been identified: 1. Instantly increasing vascular permeability due to the action of vasoactive substances. 2. Late (delayed, long) associated with the action of PMN-leukocytes. Leukocyte granules contain biologically active substances that are released during degranulation and phagocytosis. The process of accumulation of PMN-leukocytes and their degranulation is a long process. That is why they provide the 2nd phase of permeability enhancement. The increase in vascular permeability is due to the following factors: 1. The direct action of the factor (animal poisons, bacteria toxins, etc.). 2. The action of a BAS (histamine, serotonin, kinins, etc.) 3. Acidosis. It leads to liquefaction of colloids and weakening of interendothelial connections. The increased permeability of the vessels leads to the release of proteins and blood elements in the inflamed area. The release of water and substances dissolved in it is due to: 1. An increase in filtration area and diffusion. 2. Increased blood pressure in the capillaries and venules. 3. Increased osmotic pressure in the inflamed tissue. 4. Lymphatic edema. The fluid that goes into the inflamed tissue is called ekssu- date It contains a large amount of protein (30-50 g / l), blood corpuscles, cells of damaged tissue. Noninflammatory exudate - transudate, contains much less protein, blood corpuscles, cells of damaged tissue. In parallel with the release of proteins and water during inflammation, the process of leukocyte emigration proceeds. Leukocyte emigration The exit of leukocytes is preceded by the wall movement and their standing, which is observed especially clearly in the stage of venous hyperemia. This phenomenon is explained by a decrease in the negative charge of leukocytes, near-wall microcoagulation, as a result of which microfibers inhibit the movement of leukocytes and contribute to their near-wall standing. More I.I.Mechnikov noted that PMN-leukocytes appear first in the inflammation, then monocytes and the last lymphocytes. Leukocytes emigrate in two ways: PMN-leukocytes exit through interendothelial gaps, and mononuclear cells through the body of endothelial cells. The latter process is the most time-consuming and this explains why mononuclear cells later appear in the inflamed area. The basement membrane of the blood elements is overcome on the basis of an isothermal reversible decrease in the viscosity of a colloidal solution (thixotropy), i.e. transition of the gel to sol when the leukocyte is attached to the membrane. The leukocyte, easily overcoming the sol, turns out to be outside the vessel, and the membrane again turns into a gel. In this process, enzymes are involved, and above all collage-Naza. A certain influence on the sequence of emigration has the pH of the source of inflammation. At pH 7.4-7.2, PMN-leukocytes accumulate, at pH 7.0-6.8, mononuclear cells, and at pH 6.7, all leukocytes die in the nidus of inflammation to form pus. Important in the emigration of leukocytes belongs chemotak-sisu. It is formed with the participation of complement. The use of inhibitors of complement prevents damage to the vessels and the release of leukocytes. Chemotaxis is stimulated by streptokinase. Chemotoxins appear when mechanical damage tissue, with infectious inflammation due to the action of endotoxins. Chemotoxins are also formed by lymphocytes during the breakdown of gamma globulins. Chemotaxis is stimulated by metabolic products of tissues, bacteria, viruses, and the kallikrein system. A certain role in the emigration of leukocytes is played by the so-called surfactants, which can lower the surface tension. For example: organic acids. By altering the surface tension of a leukocyte, they cause the latter to develop cytoplasmic protrusions and form pseudopodia. Gradually, the entire leukocyte moves into it, entirely going beyond the vessel. The fate of leukocytes released from the vessels depends on the environment in which they fall. If the inflammation is aseptic in nature, then the emigrated leukocytes die quickly during 3-5 days. If the inflammation has a septic character, then the number of leukocytes in the inflammatory focus is progressively increasing. Suppuration begins. Some leukocytes located to the center of the inflammatory focus die. The part shows phagocytic activity. Enzyme activity is increasing: myeloperoxidase, acid hydrolases that destroy extracellularly located bacteria. Despite the fact that bacterial plaque is the primary cause of the development of inflammatory periodontal diseases, only its effect cannot explain the severity of periodontal destruction. The body's reaction plays a crucial role in the development periodontal disease. The human body has a complex set of interdependent protective mechanisms aimed at eliminating microorganisms, achieving healing and maintaining a healthy state. Paradoxically, the same system, which is designed to protect and heal the body, leads to tissue damage in periodontal diseases. Immunology is an extremely complex subject. Moreover, it is rather difficult to separate such notions as inflammatory and immune response, since in many situations their action overlaps each other. This chapter provides an overview of the manifestations of the inflammatory and immune response, as well as their role in the healing and destruction of periodontal disease. The following topics will be covered:
INFLAMMATION Inflammation is a clear sequence of events that develop in response to any damage or infection, thus, has a "non-specific" character. Inflammation is the primary response that occurs before the activation of the immune system. The process of inflammation is characterized by three stages:
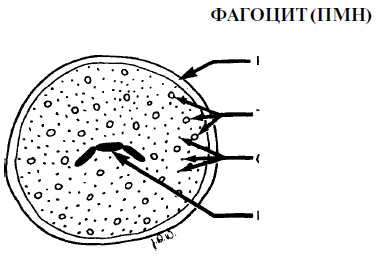
Before we start discussing the process itself, let us present the main cellular and molecular elements of inflammation. Cellular elements of inflammation The main cells responsible for inflammation are leukocytes (PMN), which are formed in the bone marrow from the same stem cells as monocytes. The specific markers of the cell surface determine which path will lead to the development of promyelocytes - along the path of formation of macrophages or PMN. These markers disappear after the completion of differentiation. The presence of several PMNs in the attached epithelium is considered normal. The increase in their number is a sign of the initiation of the reaction of the organism. PMN are phagocytes and make up 70% of the total number of leukocytes. The cytoplasm of PMN contains elements that are responsible for the movement of cells during chemotaxis activation, as well as lysosomes that destroy bacteria. The destruction of bacteria by these cells usually, but not always, occurs after the microorganisms are absorbed by PMN (ie, after phagocytosis). The next cells involved in the inflammatory response are macrophages, which are formed from circulating monocytes, and appear in the area of inflammation after PMN. Macrophages are large cells with the same phagocytic abilities as PMN. In addition, macrophages play an important role in the immune response. Lymphocytes penetrate the region of inflammation last and are associated primarily with chronic inflammation. In addition, lymphocytes are the main cells of the immune system. Mast cells are the same as circulating basophils. They release histamine, platelet activating factor (PAF), prostaglandin E2 and leukotrienes (LTB4 and LTD4), each of these elements has a pronounced inflammatory effect. Platelets release serotonin (an important mediator of inflammation). Molecular components of inflammation Histamine increases the permeability of blood vessel walls, thus facilitating the access of inflammatory cells to the affected area. Histamine is released by mast cells and basophils. Serotonin (5-hydroxy-tryptamine) also increases vascular permeability. Basophils, neutrophils and macrophages release platelet activating factor (PAF). PAF increases the release of serotonin from platelets. The neutrophil chemotaxis factor (NCF) is released from mast cells and regulates PMN chemotaxis. Chemokines are released by leukocytes. They constitute a large group of cytokines that cause mast cell degranulation and PMN chemotaxis. Warning: The terminology may seem rather confusing. All molecules that affect the immune or inflammatory response are called cytokines. In line with this, all chemokines are cytokines, but there are a large number of cytokines that are not chemokines. Activated complement SZ causes mast cell degranulation. Activated complement C5a leads to mast cell degranulation, phagocyte chemotaxis, PMN activation and an increase in capillary permeability. Bradykinin (an element of the kinin system) causes vasodilation and increases vascular permeability. Fibrinopeptides are products of the coagulation mechanism and affect the chemotaxis of PMN and macrophages. Prostaglandin E2 (PGE2) is a product of cyclooxygenase and causes vasodilation, simultaneously with an increase in vascular permeability under the action of histamine and bradykinin. Leukotriene B4 (LTB4) is formed during the lipoxygenase cycle. It stimulates the PMN chemotaxis and synergistically with PGE2 leads to an increase in the permeability of the vascular wall. Leukotriene D4 (LTD4), which also forms during the lipoxygenase cycle, increases vascular permeability. The neutrophil chemotaxis factor (NCF) is released by basophils. Selectins are a group of three molecules that facilitate the migration of PMN and macrophages through the vascular wall. Selectins E and selectins P are specific for PMN, and selectin L is for macrophages. Selectins slow down the movement of cells, which contributes to the adhesion of the latter to the vessel wall. The three groups, including the group that is known as ICAMS, include at least 12 molecules that perform similar functions. Acute inflammation in periodontal diseases As mentioned above, the process of acute inflammation includes three stages. As the bacterial plaque accumulates in the groove, the following events occur. Blood supply increases due to the expansion of blood vessels in the affected area. Some mediators cause vasodilation, for example histamine and PGE2. Serotonin, C5a, bradykinin, fibrinopeptides, PGE2, LTB2 and LTD2 increase the permeability of the vascular wall and increase the space between the endothelial cells. Selectins and ICAMS slow down the movement of PMN, allowing the latter to penetrate into connective tissue. Migration and phagocytic function of PMN are regulated by chemotactic factors, such as NCF. Chemokines, C5a, fibrinopeptides and LTB4 also contribute to neutrophil phagocytosis and chemotaxis. The main phagocytic cells that are involved in the body's response to infectious invasion are polymorphonuclear neutrophils and macrophages. The destruction of microorganisms, usually, but not always, occurs after their absorption by the cells. Damage to some cell membranes can lead to the formation and release of factors that lead to the onset of clinical symptoms of inflammation.
Eleven Classical Proteins
Granules are also called lysosomes or "suicidal packets." enzymes that destroy bacterial cells and body cells Phagocytes Oxygen-independent phagocytosis This process occurs as a result of the action of a large number of destructive substances contained in organelles that are located in the cytoplasm of phagocytes. Such organelles are called granules or lysosomes. The destructive activity of enzymes leads to the release of the contents of the granules and other factors that belong to the groups of cationic proteins, neutral proteases, acid hydrolases, as well as other substances, such as lactoferrin. Enzymes destroy bacteria after they are absorbed by phagocytes. However, in the process of phagocytosis, some enzymes can “leak” from the phagocyte and interact with the structures surrounding the cell. Probably, this phenomenon is of great importance in a groove or pocket fluid, where the destruction of bacteria begins without prior absorption, which contributes to the protection of periodontal tissues. Moreover, lysosomal enzymes can play an important role in neutralizing the action of destructive enzymes and toxins synthesized and released by bacteria, regardless of whether these enzymes and toxins were previously absorbed by phagocytes. Oxygen-dependent phagocytosis This process leads to the destruction of bacteria located inside the cell organelles, called phagolysosomes. During it, toxic oxidants and hydrogen peroxide are released from oxygen radicals and lysosomal enzyme myeloperoxidase, which leads to massive death of bacterial cells as a result of damage to their cell wall. In the course of many studies, the relationship between polymorphonuclear neutrophils (PMN) and the state of periodontal tissues has been studied. Periodontal diseases are more common and more severe in the presence of neutrophils in humans or animals, such as agranulocytosis or leukocyte adhesion failure. In animals with a decrease in the number or congenital insufficiency of PMN, rapid periodontal destruction and loss of teeth occurred. Clinical studies involving a large number of patients have shown that the presence of functional insufficiency of PMN in patients is a high risk factor for the destruction of periodontal tissues. In 1996, at the International Congress of Parodontology, Offenbacker suggested that patients with normal PMN are likely to develop gingivitis, but not periodontitis, regardless of the degree of bacterial load. On the other hand, the presence of functional insufficiency of PMN in most cases is accompanied by a loss of attachment. The findings of these studies suggest that PMNs play a crucial role in ensuring a healthy periodontal condition, but can lead to the destruction of periodontal tissues. Destruction of body tissues It is well known that the body itself leads to a significant destruction of its own tissues during the development of periodontal diseases. Such damage can be considered as a pathological reaction in the presence of chronic inflammatory disease. The following substances have the ability to destroy periodontal tissues in the process of the body's defensive reaction against bacteria and their metabolic products.
Serum complement system The serum complement system consists of more than 20 whey proteins that, when activated, possess biological activity. This system plays an extremely important role in the inflammatory and immune response. There are two main mechanisms of activation of proteins of the complement system. The first classic mechanism is activated after the antibody binds to the surface of the bacterium wall. The second alternative mechanism can be activated directly by the wall components of some gram-negative bacteria. Such components are called endotoxins. Below are just some of the many serum complement activation factors that are involved in both mechanisms.
Biologically active factors formed in the serum complement system are likely to play an important role in protecting the body against microbial invasion of periodontal tissues, as they lead to the destruction of bacteria and the initiation of other defense mechanisms that reduce the concentration of microorganisms. As is the case with all protective mechanisms when complement is activated, there is a possibility of damage to periodontal tissues. At the end of this chapter there will be a discussion of the peculiarities of damage to periodontal tissues by antibodies and phagocytes induced by complement. However, in addition, due to the activation of the complement system, the membranes of the body’s own cells, especially red blood cells, can be destroyed. Activation of complement can lead to the destruction of periodontal tissues, which determines the clinical symptoms of the disease. IMMUNOLOGY Traditionally, we consider two parts of the immune system: cellular immunity and humoral immunity. Despite the expediency of such a separation, immunologists are currently trying to characterize immune system by elements that recognize cellular antigens, and by elements that recognize free antigens. Cellular elements of the immune system
Cytokines and other molecular elements Cytokines are non-antibody molecules that have the ability to affect many components of the immune and inflammatory response, such as the compliment cascade, bradykinin, the coagulation process and the arachidonic acid cascade. The most important cytokines include:
Immunoglobulins (antibodies)
Immune response in periodontal disease With the accumulation of bacterial plaque in the area of the groove, a short period of time (usually several days) passes, during which antibodies are not detected. After a few days, the body begins to react to the presence of bacteria and their metabolic products. Fibroblasts, macrophages and lymphocytes release IL-1, IL-2, IL-6 and IL-8. Activation of selectins and 1C AM occurs, which initiates diapedesis (percolation through the vascular wall), migration and chemotaxis of polymorphonuclear leukocytes. The process of diapedesis is accelerated, and PMN is followed by macrophages. Both cell types are activated by cytokines. Clinically, this is manifested by primary redness with gingivitis. Antigens are “delivered” to B cells and monocytes using T helper cells. At last, cytokines are released. This leads to the production of B cells, which form antibodies specific for each antigen. Antigens undergo opsonization and phagocytosis, resulting in the release of substances that damage collagen and the main substance. SZa and C5a lead to the release of histamine by mast cells, this causes vasodilation and facilitates the migration to the interested area of a larger number of protective cells. Ultimately, the epithelium of the grooves ulcerate, which contributes to an even faster penetration of bacterial antigens. At this point, the gums swell, bleed and can be a little painful. Cytokines produced by fibroblasts, PMN and other cells, can play both a protective and damaging role. The affected area is infiltrated with lymphocytes and plasma cells. In the absence of treatment or in case of insufficiency of defense mechanisms, the loss of attachment occurs as a result of the action of bacteria, and as a result of the body’s response to a bacterial irritant. Conclusion In a state of health between the bacteria and the defense mechanisms of the body there is a balance. With the development of the disease this balance is disturbed, the bacteria and the body's efforts to destroy the bacteria and cure, lead to the destruction of periodontal tissues. Such an imbalance may occur as a result of the action of virulence factors, defense mechanisms or external factors, for example, under the influence of tobacco smoking. Periodontal Alphabet |
| Read: |
|---|
Popular:
Birch hanging or warty
|
New
- The program of intensive moisturizing of the skin on cosmetics bark
- What you need for acrylic powder
- What does owl mascot mean
- Analyzes for pancreatitis: what research should be done and what indicators show
- Owl - a talisman to attract money and good luck
- What bird screams at night with a kitten's voice?
- Cholesterol and stress
- Manicure at home
- Effective facial
- What is a man after a broken leg?